![]()
Prevention is better than Treatment
R&D's
GALMMR2000 Screening Kit
- It couldn't be easier
R&D
DIAGNOSTICS LTD®
GALMMR2000
ASSAY (550 nm)
Available in boxes of 2000 tests (5X400).
· Take a clean 96-well (preferably U bottom) microplate (elution microplate).
· Add one disk cut from a dried blood spot (3/16 inch or 2 X 1/8inch diameter) per well. Remember to add controls, standards and one blank well.
· Warm up all reagents to room temperature.
· Add 100 microliters of Elution Buffer (TCA 3%) in each well, mix well the contents of each well and place the plate on a plate shaker.
· Wait 30 minutes at room temperature (20-26oC). While waiting reconstitute one Coenzyme Vial with 38,4 ml of distilled water. The Dilution buffer, Enzyme 1 and Enzyme 2 reagents are ready to use. Mix the four reagents according to the table below in correlation to the number of tests to be run. Shake well the enzyme vials to resuspend all matter. We strongly recommend that the dilution buffer be added to the mixture just before use. You need 100 microliters of this Enzyme1-Enzyme2-Coenzyme-Dilution buffer mixture for each sample. Please note that you should only mix the quantity you need for the day's run. The Enzyme1-Enzyme2- Coenzyme mixture should be discarded if not used within 8 hours.
· Transfer 40 microliters of the TCA eluant in a new microplate at the corresponding wells. Add 100 microliters of the mixture prepared in the previous step per well. Mix well, avoiding the formation of foam. Wait for 30 minutes at room temperature (20-26oC)
· Take the Color Reagent and the Color Reagent Booster out of the refrigerator; mix one part of Color Reagent Booster with 10 parts color reagent just before using it. Do not pre-warm the mixture. Return the original bottles back to the refrigerator the soonest possible. Avoid exposure to light. Prepare only the quantity you will need for the day.
· Add 100 microliters of the Color Reagent Mixture per well. Mix well avoiding the formation of foam. Always bring it back to the refrigerator when the assay is over.
· Wait for 10 minutes and measure the microplate at 550 nm, endpoint mode, single measurement. Do not wait longer than 15 minutes. If this stage is prolonged (i.e. more than 20 minutes) a high background may be observed.
· Calculate the slope and the sample values.
|
# tests |
enzyme I microliter |
enzyme II microliter |
Coenzyme in ml |
dilution buffer in ml |
total volume in ml |
|
50 |
10 |
182 |
3,845 |
1 |
5 |
|
100 |
20 |
365 |
7,68 |
2 |
10 |
|
150 |
30 |
546 |
11,52 |
3 |
15 |
|
200 |
40 |
729 |
15,36 |
4 |
20 |
|
250 |
50 |
911 |
19,2 |
5 |
25 |
|
300 |
60 |
1092 |
23 |
6 |
30 |
|
350 |
70 |
1274 |
26,93 |
7 |
35 |
|
400 |
80 |
1461 |
30,73 |
8 |
40 |
|
450 |
90 |
1643 |
34,56 |
9 |
45 |
|
500 |
100 |
1825 |
38,4 (vial) |
10 |
50 |
|
1000 |
200 |
3650 |
76,8 |
20 |
100 |
|
2000 |
400 |
7300 |
153,6 |
40 |
200 |
|
Contents:
Enzyme I : 1 vial X 110 microliters (red plastic cover) - orange label
Enzyme II : 1 vial X 1,85 ml (transparent clear glass vial) - orange label
Coenzyme : 5 vials X 7,7 ml (freeze dried powder reconstitute with distilled water) red label
Dilution buffer : 1 vial X 12 ml - green label
Color Reagent : 1 vial X 44 ml - yellow label
Color Booster : I vial x 4,5 ml – black label
Brief instructions for use
One set of standards / controls of Galactose on special filter paper (see attached document for values & exp. Date)
Keep refrigerated when not in use - do not freeze.
GAL reaction :
â-D-Galactose -1- Phosphate à â-D-Galactose
(catalyzed by Alkaline Phosphatase)
â-D-Galactose + NAD+ + H2O à D-Galactono [1,4] lactone + NADH+ +H+
(catalyzed by Galactose dehydrogenase; measured at 340 nm)
NADH+ + Color reagent (bright yellow) à Color Reagent (blue/violet) + NAD
(catalyzed by Diaphorase; measured at 550 nm)
The color reagent in its reduced form [ Blue ] will stain glass or plastic after prolonged contact. It is advisable to discard such material after the test is over, especially when the microplate or other equipment is to be re-used
INSTRUCTIONS FOR USE (PACKAGE INSERT)
|
An Enzymatic Colorimetric Assay for the Quantitative Determination of Total Galactose (Galactose and Galactose-1-Phosphate) Levels In Neonates. A SCREENING KIT FOR DRIED BLOOD SPOTS This kit is particularly suitable for screening for Galactosemia in newborns. KIT SIZE: 2000 TESTS
MANUFACTURER CODE: GRM7 - EDMA CODE: 11.02.01.12 - PRODUCT FILE No. GR/CA01/GRM7/O/125 R&D Diagnostics Ltd 33, Alevizatou street 15669 Papagos Greece Website: www.rddiagnostics.com
Version IV, 01/09/2004 Intended Use The R&D Diagnostics Total Galactose Kit is an Enzyme Assay (EA) for the quantitative measurement of total galactose (galactose and galactose-1-phosphate) concentrations in dried blood spot samples that have been collected onto Schleicher & Schuell (S&S®) 903™ specimen collection paper. This kit is particularly suitable for use in a newborn screening program to measure total galactose concentrations in newborn infants as an aid for the early detection of Galactosemia. Clinical UtilityGalactosemia is a disorder caused by an inborn error of galactose metabolism. The rate of incidence is approximately 1 in 50,000 newborns worldwide.1 Galactosemia is an autosomal recessive disorder that is characterized by elevated concentrations of galactose in the blood resulting from the absence or dysfunction of any of the three enzymes responsible for the transformation of galactose to glucose, i.e., D-galactose-1-phosphotransferase (EC 2.7.1.6), a-D-galactose-1-phosphate-uridyltransferase (EC 2.7.7.12) or UDP-glucose-4-epimerase (EC 5.1.3.2).2 The symptoms associated with Galactosemia in the newborn period can include vomiting, diarrhea, dehydration, jaundice, hepatic failure, hypoglycemia, cataracts, and developmental retardation.3 Sepsis due to Escherichia coli has frequently been the cause of death in neonates diagnosed with Galactosemia.4 The increased circulating concentrations of galactose, if left untreated, can result in a variety of symptoms including metabolic cirrhosis of the liver, mental retardation, cataract formation, and kidney damage. Treatment consists of removal of galactose and its major precursor, lactose, from the diets of affected individuals. This galactose-free diet should be initiated as early as possible and maintained throughout life.5 Principle of the AssayThe R&D Diagnostics Total Galactose Kit uses trichloroacetic acid (TCA) to extract total galactose (galactose and galactose-1-phosphate) from dried blood spot samples. After extraction the eluted sample is combined with an Enzyme-Coenzyme solution containing galactose dehydrogenase (EC 1.1.1.48), alkaline phosphatase (EC 3.1.3.1) and nicotinamide adenine dinucleotide (NAD). Galactose-1-phosphate is converted to galactose by the enzymatic action of alkaline phosphatase. Galactose is oxidized by galactose dehydrogenase in the presence of NAD and results in the production of galactono-lactone and NADH.6 The amount of galactose in the dried blood spot sample can be quantitated by measuring the reduction of NAD to NADH. The amount of NADH produced is measured colorimetrically by the conversion of the colorless tetrazolium salt into a colored formazan. The intensity of this reaction product can be measured with a microplate reader at a dual wavelength setting of 550 nm and is directly proportional to the concentration of galactose present in the sample. Materials Supplied 2000 Tests / KitGalactose Standards and Controls 1 Set Galactose Enzyme I 1 x 0.44 ml Galactose Enzyme II 1 x 7,3 ml Dilution Buffer 1 x 42 ml Coenzyme (Lyophilized) 4 x 39 ml Color Reagent 1 x 175 ml Color Reagent Booster 1 x 18 ml TCA (may not be provided) 4 x 52 ml Reagent DescriptionGalactose Standards and ControlsHuman whole blood which has been adjusted to a hematocrit of 55% and contains five standard concentrations of added galactose, i.e., approximately 2.0, 5.0, 10.0, 25.0 and 50.0 mg/dl. The three Controls contain low, mid and high concentrations of galactose. The Standards and Controls are spotted onto Schleicher & Schuell (S&S®) 903™ specimen collection paper. Refer to the labels for the exact concentrations of the Standards and the acceptable ranges for the Controls. Storage: Dry at 2-8º C Expiration: Refer to the expiration date printed on the label Galactose Enzyme IAlkaline phosphatase. Shake well before use. Storage: 2-8º C Expiration: Refer to the expiration date printed on the label. Galactose Enzyme IIGalactose dehydrogenase. Shake well before use. Storage: 2-8° C Expiration: Refer to the expiration date printed on the label. Dilution BufferA solution containing buffers, stabilizers and 0.01 % (w/v) sodium azide (NaN3) added as a preservative. Storage: 2-8° C Expiration: Refer to the expiration date printed on the label. CoenzymeLyophilized NAD with buffers and stabilizers. Storage: 2-8º C Expiration: Refer to the expiration date printed on the label. Stable for 1 month at 2-8º C after reconstituting. Color Reagent
A solution of a tetrazolium salt and a buffer Storage: 2-8º C / Keep away from light Expiration: Refer to the expiration date printed on the label. Color Reagent Booster A solution of an intermediate electron receptor and buffer Storage: 2-8º C / Keep away from light Expiration: Refer to the expiration date printed on the label. TCA (may NOT be provided)A 3.0 % (w/v) solution of trichloroacetic acid (TCA). Storage: 2-8º C Expiration: Refer to the expiration date printed on the label. Materials required:
· Pipets; 40 ml, 100 ml, 16 ml dispenser · Controls, Standards, Bloodspots (1 Spot 3/16 inch or 2 x 1/18 inch ) · TCA 3% (may be provided) · Distilled water · Plateshaker Assay Procedure1. Take a clean 96-well (preferably U bottom) microplate (elution microplate). 2. Add one disk cut from a dried blood spot (3/16 inch or 2 X 1/8inch diameter) per well. Remember to add controls, standards and one blank well. 3. Equilibrate all reagents to room temperature. 4. Add 100 ml of Elution Buffer (TCA 3%) to each well, mix the contents of each well and place the plate on a plate shaker. 5. Incubate 30 minutes at room temperature (20-26 oC). 6. Reconstitute one Coenzyme Vial with 39 ml of distilled water. The Dilution buffer, Enzyme 1 and Enzyme 2 reagents are ready to use. Mix the four reagents according to the table shown in the brief assay included in this kit in correlation to the number of tests to be run. We strongly recommend that the dilution buffer be added to the mixture just before use. You need 100 ml of this Enzyme1-Enzyme2-Coenzyme-Dilution buffer mixture for each sample. Note that you should only mix the quantity you need for the day's run and the Enzyme1-Enzyme2- Coenzyme mixture should be discarded if not used within 8 hours. 7. Transfer 40 ml of the TCA eluate in a new microplate at the corresponding wells. Add 100 ml of the mixture prepared in the previous step per well. Mix well, avoiding the formation to foam. Incubate for 30 minutes at room temperature (20-26 oC) 8. Take the Colour Reagent out of the refrigerator. Take the quantity you will need for the numbers of test (assuming 80 microliters / sample) and the Color Reagent Booster (10 parts Color Reagent : 1 part Booster). Add 80 ml of Colour Reagent per well. Mix well to avoid the formation of foam. Place back the Colour Reagent and the Booster in to the refrigerator when the assay is over. 9. Incubate for 10 minutes and measure the microplate at 550-570 nm (optimum 550 nm), endpoint mode, single measurement. Do not wait longer than 15 minutes. If this stage is prolonged (i.e. more than 20 minutes) a high background may be observed. 10. Calculate the slope and the sample values.
|
|
|
Volumes required from each of the four components to run specific number of tests (volumes in ml; volumes for the enzymes is expressed in ml).
* The color reagent in its reduced form [ Blue ] will stain glass or plastic after prolonged contact. It is advisable to discard such material after the test is over, especially when the microplate or other equipment is to be re-used
Gal Reaction:
(catalyzed by Alkaline Phosphatase)
(catalyzed by Galactose dehydrogenase; measured at 340 nm)
(catalyzed by Diaphorase; measured at 550 nm)
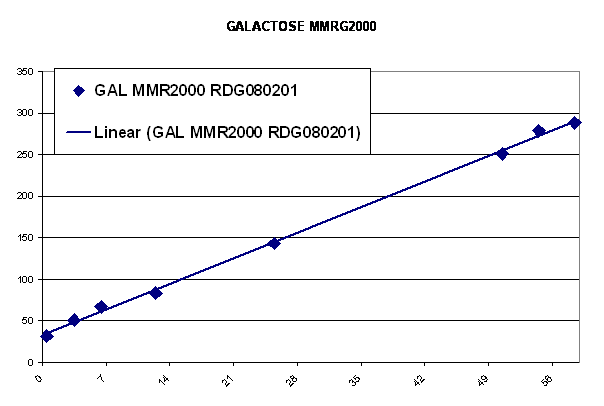
The following graph shows the responses (in mOD) of standards with known Galactose content (expressed in mg/DL in the x-axis). Thirteen samples containing different concentrations of galactose were tested in triplicates and the average mOD values were plotted against galactose concentration. The red trendline shows the results of a kit commercially available (samples tested in duplicates).
The R&D Diagnostics Ltd Total Galactose Kit is intended for use as a tool to screen neonates for elevated levels of total galactose (galactose and galactose-1-phosphate). This Kit is not to be used for confirmatory testing or to monitor therapy. A definitive clinical diagnosis should not be based on the results of a single test but should be made by the physician only after all clinical and laboratory findings have been evaluated. Another diagnostic procedure performed on a serum sample should be used to confirm the diagnosis of Galactosemia.
Infants that have not ingested breast milk or formula containing lactose prior to the sample collection may give low total galactose values. Exchange transfusions may also lead to false negative results.9
Excessive pressure applied to the puncture site can cause hemodilution of the blood sample and result in an apparent decrease in total galactose concentration.
Precautions
1. All blood samples of human origin should be regarded as a potential Biohazard. Handle all blood samples as if capable of transmitting Hepatitis and HIV.
2. The Enzyme Diluent, Color Reagent, Color Reagent Booster, Standards and Controls all contain sodium azide (NaN3) and Proclin 300 as antimicrobial preservatives. Users should be aware of their toxic properties if absorbed or ingested. Disposal of these reagents should be accompanied by copious flushing with water to avoid accumulation of explosive salts in plumbing systems.
2. The blood spot eluent solution, trichloroacetic acid (TCA), is highly acidic and corrosive. Protective gloves should be worn while using this reagent.
3. Do NOT keep or use the reconstituted Enzyme, Coenzyme, or the combined Enzyme-Coenzyme working solution for any longer than the specified periods of time.
4. All of the Kit components used in an assay must be from the same Kit Number.
5. Kit components should not be used past the expiration dates printed on the labels.
6. Kit components and test specimens should be at room temperature (18-25º C) before starting the assay. The color reagent is the exception to this rule. Do not warm it before use.
7. Do not use any reagents or solutions that have become cloudy or discolored. This is especially true for the color reagent. The color reagent should be colored yellow. Do not use it if a blue tint is observed.
The reproducibility of the standard curve values and control values should be within defined limits of laboratory acceptability. Commonly used measures of variability are discussed by Westgard, J.O., et al.10 If the precision of the assay does not correlate with this standard and repetition excludes errors in technique, check the following areas:
a. Pipetting and timing devices
b. Instrument calibration
c. Expiration dates on reagent labels and prepared working solutions
d. Storage conditions
e. Temperature control devices
About us The Disorders The Products Kit Inserts Literature Technical Support Articles Events Links Contact us To Order
Hosted by
ohmywebsite.com
TO
SEARCH THIS SITE - CLICK
SEARCH
Page last edited on 21/03/2006