![]()
Prevention is better than Treatment
|
G-6-PD Frequently Asked Questions (FAQ)
Question
1. Is G-6-PD neonatal screening not enough? Question
2.
What happens to the U/g Hb values when screening for
G-6-PD at different temperatures? Question
3.
Sometimes the first kinetic readings are not linear. Why is
that so? How do we compensate? Question
4. For how long after collection are the Dried Blood Spots
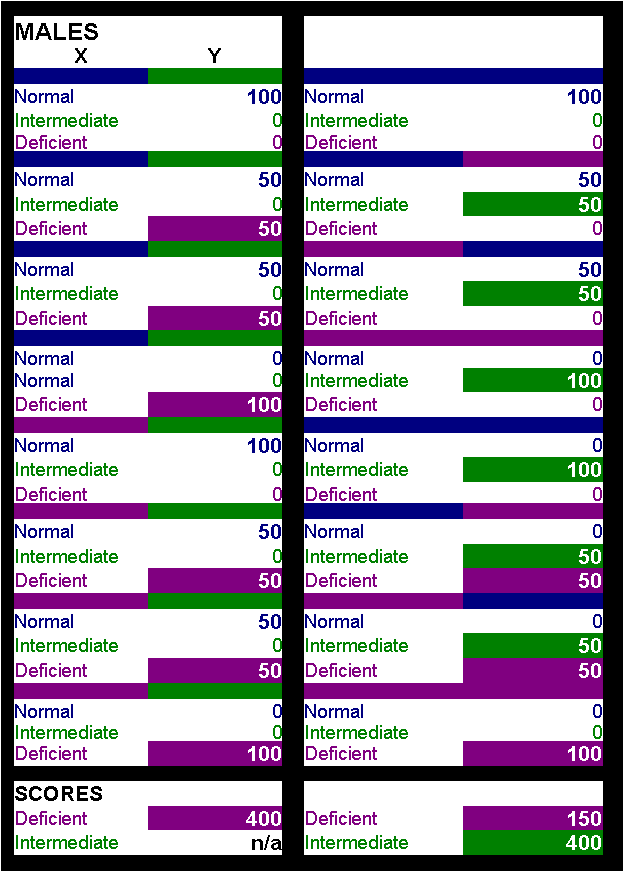
stable? Question 5. What are the drawbacks of the "classic" Beutler method? Why is Quantitative analysis so important? One can screen the population and then go for the quantitative analysis of the positive samples only, isn't it? Isn't cost prohibitive? Answer: Too many questions without easy answers, but closely related. To answer it in a step by step manner we would say that for the classic Beutler method the disadvantages are : time consuming, temperature restricted and very little information for the clinician. To us the only real advantage of this method is cost. However, a careful economic analysis may prove that even this is not so. In countries where the disease is endemic this is definitely not true. When a population of 100.000 newborns is screened by this method and then you have to analyze at least 10.000 of them quantitatively then even cost is not in favor. Calculating the extra time consumed, the extra labor needed, the extra 10.000 tests (at more than 1 pound per test), the transfer of the samples and all the alike, it seems that doing it quantitatively in the first place with a reasonably priced kit (like the MMR500 test kit) may be more economic than you think. Moreover, the method can't pick up the Intermediate (partially deficient) cases. If one examines closely the statistical data for Greece it is clear that G6PD deficient boys are many more than girls (4.5% vs 1.84%). However, (if we are allowed a simplification of the situation) the possibility of acquiring the deficient X chromosome is the same for both sexes. The only difference is that boys carrying this X chromosome are heavily deficient whilst girls having only one deficient X chromosome are partially deficient, something clearly not shown by the Beutler method. Instead, all partially deficient samples are taken as normal - which clearly is NOT the case. Recently we were lucky enough to have a whole family with a history of G-6-PD deficiency. The grandmother had an activity of 4.2 U/g Hb (intermediate), the mother 4.0 U/g Hb (intermediate), the father 11.2 U/g Hb (normal), the daughter 12,1 U/g Hb (normal) and the son 0,2 U/g Hb (deficient) as measured with our reagents. These results tell the whole story concerning the origin of the deficient chromosomes and supply enough information to the clinician. However, with the Beutler method only the boy was detected as Deficient. To support our case, the mother (aged 39) reached us after an "unexplained" hemolytic episode following drug medication. Ý Top Ý Question 6: a) From the assay description on your site it is not clear as to the mechanism by which the test measures the enzyme. What is the label of the y-axis? Is the test a variant of the dye-reduction test the only difference being the quantity of blood needed (a spot versus several ml of whole blood)? b) It is not clear as to what equipment is actually included in the kit; is the microtiter plate included? c) is there a way for an ordinary citizen (a.k.a. someone without an orbital shaker and incubator) to perform the test successfully and without an error? (asked by Mrs. Glynda Mercier, Texas, USA) Answer(s) : a. The y-axis is OD (Optical Density), the x-axis is time (in minutes). The test has nothing to do with the widely used dye reduction system. Although the principle behind all these tests is the famous work of Beutler still there are many ways to approach this test. In our system we use the reaction which is shown in our website (see bottom of this message to save some time). The reaction is straight forward and it resembles exactly the reaction that takes place in our bodies. The quantity which is measured DIRECTLY is the beta-NADPH which is produced. The enzyme (G6PD) converts NADP to NADPH stoichiometrically (through the conversion of G6P) and we measure the quantity of NADPH produced at 340 nm (photometrically). Since we are speaking about an enzyme we are definitely talking about a kinetic reaction. Which is, the main issue is "how much substrate is converted by the enzyme per time unit" which is translated to "how much NADPH is produced per time unit". Since you seem to know a lot about these things we can simplify the method a little and say that "any device capable of measuring at 340 nm over a specific period of time can give us the answer". It can be a microplate reader, a simple photometer or a $$$ automated analyser. All you need to know is how much did the OD changed at 340 nm over a specified amount of time. You can take two measurements using a simple spectrophotometer ten minutes apart and then divide the delta OD by ten in order to get the "change of OD per minute" entity which is what you are looking for. All you need is a control with known activity to compare against (many in the market). One catch though. When comparing the activity of your blood to the control you have to refer to the same hemoglobin content. In other words we need to know how much enzyme activity was measured using the same number of red blood cells. This calls for a hematocrite evaluation of your blood and the control before doing out test. I would also like to answer your question about the quantity of the blood actually needed. Our method needs 5 microliters of whole blood to work (I am sure you know what micro-something is). Of course you will have to get a hematocrite evaluation but this consumes less than 1 ml, so we can say that 1 ml of whole blood covers it. If you know your hematocrite then even 1 drop of blood is too much. Of course the test will work with dried blood spots as well. b. No, the microtiter plates are not included. There is no reason for it. Since nothing is attached or linked to the microplate, one can wash and use the same plate thousands of times. On top of this, we are also producing a single test kit. Adding a microplate to this kit has no meaning. The single test kit is exactly what the name implies. Ten vials each containing chemicals for one determination. Again you can use whatever equipment you have - provided you adapt the test to the equipment (change volumes etc). c. There are two answers to your last question. The official one and the unofficial one. The official one says that only an experienced person can run the test in a fully equipped lab and... The unofficial answer says that YOU (based on your knowledge which is far above average) could do it. In theory, with whole blood you wouldn't even need a shaker because the Red Blood Cell lysis buffer works instantly, so you can gently swift the microplate or vial a minute or two and it is done. In theory, you wouldn't even need an incubator. As long as the ambient temperature is over 24 degrees (Celsius) you can do this test on the bench. Since the control you will be using will be calibrated at 37 degrees but it will run at the same temperature (e.g. 24 degrees) all you need is to compare the activity of your blood to the control. If for example you observe that your blood contains only 75% of the control's activity at any given temperature then it would do the same at any temperature including 37 degrees (most controls are calibrated at this temperature). Provided you have the hematocrite values you have an answer at home.... However, I must confess that if something goes wrong you will not be in a position to counteract. Ý Top Ý Question 7: Is it true that "Internediate" samples are not "found" with the semi-quantitative methods ? What does the "Intermediate" level correspond to? Isn't it safe to be an "Intermediate"? Answer: We will start the answers in reverse order. Unfortunately, being classified as an intermediate stage G6PD person is far from safe. According to Scriver et al., this situation may lead to hemolytic episodes, even severe ones. This stage corresponds to people that have a limited amount of enzyme activity (10-60% of the activity found in normal subjects) and it corresponds to the heterozygote females. Though this genetic metabolic disorder was thought to be an X-linked recessive disorder it was actually found to be X-linked but not recessive. Individual studies performed by E.Beutler and Davidson et al., revealed that actually in the heterozygotes there are two different coexisting cell populations, one which has G6PD activity (G6PD+) and one which hasn't (G6PD-). It was further showed that this was because this mosaicism was genetically determined in somatic cells by faithful maintenance of the active state of one or the other X chromosome in each cell and its progeny. On top of that, the expression of the enzyme by itself is not the only factor that determines the severity of each case. Within the intermediate enzyme activity group there are sub classifications as to the specific affinity of the enzyme present to the substrate (G6P). The higher the affinity the better the response. This means that the enzyme, even though present in inadequate quantities, strongly reacts with the substrate thus exerting its protective role. In contrast, the same enzyme quantity with low affinity for the substrate leads to a more severe deficiency. Therefore, the answer it that the intermediate G6PD deficiency is far from safe and that these people should also be warned to avoid certain chemicals, foods (mainly fava beans) and drugs. Are these samples identified as "positive" during screening with the semi-quantitative method? A semi-quantitative method works using a cut-off value. Values either side of this point are characterized accordingly. The method most commonly used employs a visual examination of the samples and a subjective evaluation of the fluorescence of the samples. Apart from the "subjective" nature of the technique the main question is : what is regarded as negative. It is a fact that in all countries which use this method the positive samples of boys are almost three times more than that of girls (in fact they have a ratio of 4 to 1,5). This is also true for Greece as you can see from the relevant statistics. However, the disease is genetically inherited and as such, it follows certain rules that can be easily calculated. To start with, if a mother has a deficient X chromosome both boys and girls have the same chance of getting it, therefore the frequency of deficiency should be the same among the two sexes (the severity of the deficiency wouldn't). Since we are now discussing the frequency of G6PD deficient people (severe and intermediate deficiency) this should be the case. Therefore the 4 : 1,5 ratio can't be explained unless the test misses something. Things are even worse. Boys can't inherit the deficient X chromosome from their fathers, but girls can. So, the frequency of deficient girls should be even higher than the boys and this can't be disputed, it is how nature works. If we take into account the (wrong) fact that the frequency of deficient males is much higher than females than far more girls have a chance to acquire an X deficient chromosome which the boys can't possibly get. If you now take a look at the following simplified table of genetic combinations you will see that the 4 : 1,5 ratio found by most screening labs corresponds to the severe deficient cases ONLY. In other words only homozygote girls are identified as positive for G6PD. It is not a mistake of the labs. It is just a limitation of the technique which uses a very low cutoff point, thus classifying all intermediate samples as normal. If a quantitative test (such as our kit) was used, then the ratio should become girls : boys = 5.5 : 4 In short, the number of girls classified as deficient (severe or intermediate deficiency) should be almost 4 times higher than it is now. In other words, the current test misses 75% of the positive girls.Ý Top Ý |
|
Question
8:
I have no trace of G6PD and I am told that there are certain
foods that I can avoid. However, my doctor didn't mention anything.
What is the right thing to do? Foods to be avoided. Even the so called "Father of History" Herodotus, referred to fava beans when he wrote that the Priests in ancient Egypt prohibited the consumption of fava beans. So, fava beans should be avoided in general by G6PD patients. In our days all foods and drinks are obliged to list their ingredients on their labels. You should check to see if they contain any substance which is included in contraindicated drugs. To our surprise, even tonic water contains quinine - a substance clearly listed as contraindicated. You should also avoid contact with mothballs (contain naphthaline). Ý Top Ý Question
9: According to the data presented in your site it seems that
this test is particularly suitable for microplate readers. Is this
the only way to perform the test ? What about automated analyzers or
spectrophotometers ? Question
10: What is the impact of G-6-PD Deficiency (if any) on the
mental and physical development of a child ? (asked by Eric
Loh & Helen Lim, Sembawang, Singapore) Question
11: How can I be sure that I am not G-6-PD deficient if not
screened as a newborn ? If it turns out that I am, is there a way to
"measure" how bad my deficiency is ? 1.
Elevated bilirubin levels To ensure that these findings are due to G-6-PD deficiency a fully quantitative measurement of the enzymatic activity should be performed. This can be done using Quantase MMR010 single test kit. This will tell you (in less than 15 minutes) if your are a G-6-PD deficient patient or not. If you are, then you have to learn the drugs and foods that should be avoided and be ready to inform your doctor about your deficiency before he prescribes any drugs to you. There is a list of additional tests (very specialized though, not available everywhere) which will provide additional information on the severity of the deficiency and (perhaps) indicate the factor responsible for it. These tests include: 1.
GSH stability test Question
12: What
is the difference between a G6PD carrier and a partial G6PD
deficient patient? Can you test a person's degree of G6PD
deficiency? In other words, can you determine through blood test
if a person is (A) carrier, (B) deficient, (C) partial
deficient, (D) class I OR II OR III deficient? Answer: Well, to answer your questions: There is no such a thing as a "G-6-PD carrier" at least not in the way this term is generally used and understood. A "carrier" is a person or organism which has a defected (or mutated) chromosome which is recessive and therefore not expressed. In this case, although it exists in the organism's genome, it doesn't cause a disease. However it can be carried on to the offspring (inherited) that this is why the person is called a carrier. In G-6-PD, both the normal and the defected chromosomes are expressed (the defected chromosome is not recessive), which leads to two distinct populations one being G-6-PD+ and the other G-6-PD- Therefore, the "carrier" is always a partially deficient patient, the deficiency's severity varying a lot. The "deficient" or better still "totally deficient" patient is one that has less than 20% residual activity as compared to normal subjects. A partially deficient patient is one whose residual activity lies between 20 and 60% of the normal activity. Of course, a patient with 58% residual activity is in a much better position than a patient with 21% residual activity which makes sense. Thus we further divide deficiency in classes (I, II and III). Moreover, many mutations that lead to G-6-PD deficiency have been found and we now know that a specific mutation will lead to a specific class of deficiency. Deficiency and the degree of the deficiency are not proportional to the amount of the enzyme that the red blood cells have. It is proportional to many factors that are related to the G-6-PD produced by the defected chromosome. Thus, a person may produce very few molecules per cell but these molecules have a very high affinity for the substrate (can therefore protect the patient much better) while another may produce many more molecules of G-6-PD with a much lower affinity for the substrate. That is why we measure enzymatic activity and not the molecules themselves (which would be much easier and much more accurate). And this is the reason the patients are classified according to their enzymatic activity and not the G-6-PD content of their cells. Of course, a fully quantitative test can discriminate between these classes of deficiency. Ý Top Ý |
About us The Disorders The Products Kit Inserts Literature Technical Support Articles Events Links Contact us To Order
Hosted by
ohmywebsite.com
TO
SEARCH THIS SITE - CLICK
SEARCH
Page last edited on 05/07/2005