![]()
Prevention is better than Treatment
Glucose 6 phosphate dehydrogenase deficiency (G-6-PD)
General Information
Glucose-6-phosphate dehydrogenase (G-6-PD) is a cytoplasmic enzyme that is distributed in all cells. It catalyses the first step in the hexose monophosphate pathway, producing NADPH (Picture 1). This coenzyme is required as hydrogen donor for reactions of various biochemical pathways as well as for the stability of catalase and the preservation and regeneration of the reduced form of glutathione. Catalase and glutathione are both essential for the detoxification of hydrogen peroxide, therefor the defence of cells against H2O2 ultimately and heavily depends on G-6-PD. The red cells are exquisitely sensitive for oxidative damage and lack other NADPH-producing enzymes. The defence against oxidizing agents, epitomised by H2O2, is mainly realised by glutathione, which converts H2O2 to H2O stoichiometrically via glutathione peroxidase. NADPH is the hydrogen donor for the regeneration of reduced glutathione. An alternative pathway of H2O2 detoxification is via catalase, but this route is regarded ineffective under normal conditions (1), because of the lower affinity of catalase for H2O2 compared to that of glutathione peroxidase. G-6-PD deficiency is the most common known enzymopathy with around 400 million people affected worldwide. The prevalence ranges from 5 to 25% in endemic areas, such as Africa (2,3), the Midle East, Asia (4,5,6,7), the Mediterranean (8,9,10) and Papua New Guinea. The highest incidence is found in Kurdish Jews: 65%. Incidences ranging from 0.5 to 6.9% have been reported in North and South America. Around 400 mutations have been reported so far (11,12,13,14).
The clinical manifestations associated with G-6-PD deficiency are:
Drug induced hemolysis: certain antimalarials, sulfonamides, sulfones and other drugs or chemicals are associated with significant hemolysis in subjects (15,16,17).
Infection induced hemolysis: numerous bacterial, viral and rickettsial infections have precipitated hemolysis, but the most important are infectious hepatitis, pneumonia and typhoid fever (18,19,20).
Favism: sudden onset of acute hemolytic anemia within 24 to 48 hours of ingesting fava beans (21,22, 23, 24,25,26,28,28).
Neonatal jaundice: jaundice usually appears by 1 to 4 days of age (29,30,31,32).
Chronic nonspherocytic hemolytic anemia (33).
The reaction that goes on is the following:
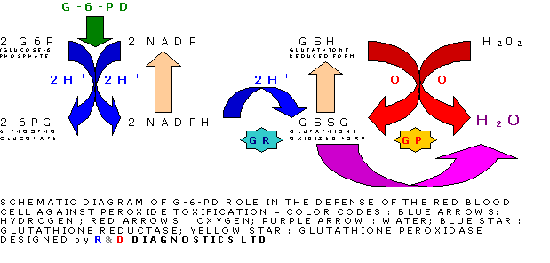
1.Cohen G. and Hochstein P. Generation of hydrogen peroxide in erythrocytes by haemolytic agents. Biochemistry, 3:895, 1964.
2. Capps F.P.A., Gilles H.M., Jolly H. and Worlledge S.M. Glucose-6-phosphate dehydrogenase deficiency and neonatal jaundice in Nigeria. Their relation to the prophylactic use of vitamin K. Lancet, ii:379, 1963.
3. Bienzle U., Effiong C.E. and Luzzatto L. Erythrocyte glucose-6-phosphate dehydrogenase deficiency (G6PD type A-) and neonatal jaundice. Acta Paed Scand 65:701, 1976.
4. Lai H.C. Lai M.P.Y. and Leung K.S. Glucose-6-phosphate dehydrogenase deificiency in Chinese. J Clin Pathol 21:44, 1968.
5. Lu T-C, Wei H. and Blackwell R.Q. Increased incidence of severe hyperbilinurinaemia among newborn Chinese infants with G6PD deficiency. Pediatrics 37:994, 1966
6. Flatz G., Sringam S., Premyothin C., Penbharkkul S., Ketusingh R. and Chulajata R. Glucose 6-phosphate dehydrogenase deficiency and neonatal jaundice. Arch Dis Child 38:566, 1963.
7. Phornphutkul C., Whitaker J.A. and Woramthumrong N. Severe hyperbilinurinemia in Thai newborns in association with erythrocyte G6PD deficiency. Clin Pediatr 8:275, 1969.
8. Doxiades S.A. and Valaes F. The clinical picture of Glucose 6-phosphate dehydrogenase deficiency in early infancy. Arch Dis Child 39:545, 1964.
9. Doxiades S.A., Fessas P.H. and Valaes F. Erythrocyte enzyme deficiency in unexplained kernicterus. Lancet ii:44, 1960.
10.Meloni T., Cagnazzo G., Dore A. and Cutillo S. Phenobarbital for prevention of hyperbilinurinemia in Glucose 6-phosphate dehydrogenase deficient newborn infants. J Pediatr 82:1048, 1973.
11.Matthay K.K and Mentzer W.C. Erythrocyte enzymopathies in the newborn. Clin Haematol 10:31, 1981.
12.Malluh A.A., Imseeh G., Abu-Osba Y.K.and Hamdan J.A. Screening for Glucose 6-phosphate dehydrogenase deficiency can prevent severe neonatal jaundice. Ann Trop Paed 12:391, 1992.
13.Solem E. Glucose 6-phosphate dehydrogenase deficiency : An easy and sensitive quantitative assay for the detection of female heterozygotes in red blood cells. Clin Chim Acta 142:153, 1984.
14.Solem E., Pirzer C., Siege M., Kollman F., Romero - Savaria O., Barktsch - Trefs O. and Kornhuber B. Mass screening for Glucose 6-phosphate dehydrogenase deficiency. Improved fluorescent spot test. Clin Chim Acta 152:135, 1985.
15.Dern R.J., Beutler E. and Alving A.S. The haemolytic affect of primaquine. II. The natural course of the haemolytic anaemia and the mechanisms of its self-limiting character. J Lab Clin Med 44:171, 1954.
16.Beutler E., Dern R.J. and Alving A.S. The haemolytic effect of primaquine. IV. The relationship of cell age to hemolysis. J Lab Clin Med 44:439, 1954.
17.Beutler E. The hemolytic effect of primaquine and related compounds. Blood, 14:103, 1959.
18.Chan T.K., Chesterman C.N., McFadzean A.J.S. and Todd D. The survival of Glucose 6-phosphate dehydrogenase deficient erythrocytes in patients with typhoid fever on chloramphenicol therapy. J Lab Clin Med 77:177, 1971.
19.Burka E.R., Weaver Z. and Marks P.A. Clinical spectrum of hemolytic anemia associated with Glucose 6-phosphate dehydrogenase deficiency. Ann Intern Med 64:817, 1966.
20.Owusu S.K., Addy J., Foli A.K., Janosi M., Konotey - Ahulu F.I.D. and Larbi E.B. Acute reversible renal failure associated with Glucose 6-phosphate dehydrogenase deficiency. Lancet 1:1255, 1972.
21. Fermi C. and Martinetti P. Studio sul favismo. Ann.Igiene Sper, 15:76, 1905
22.Luisada L. Favism: A singular disease affecting chiefly red blood cells. Medicine, 20:229, 1941
23.Sansone G., Piga A.M. and Segni G. Il Favismo, Torino, Italy, Minerva Medica, 1958.
24.Kattamis C.A., Kyriazakou M. and Chaidas S. Favism : Clinical and biochemical data. J Med Genet 6:34, 1969.
25.Kahn A., Marie J., Desbois J.C. and Boivin P. Favism in a Portuguese family due to a deficient Glucose 6-phosphate dehydrogenase variant of Canton or Canton-like type 1. Acta Haematol 56:58, 1976.
26. Belsey M.A. The epidemiology of favism. Bull WHO 48:1, 1973.
27.Szeinberg A., Sheba C., Hirschorn N. and Bodonyi E. Studies on erythrocytes in cases with past history of favism and drug induced avute hemolytic anemia. Blood 12:603, 1957.
28.Gross A.T., Hurwitz R.A. and Marks P.A. An hereditary enzymatic defect in erythrocyte metabolism. Glucose 6-phosphate dehydrogenase deficiency. J Clin Invest 37:1176, 1958.
29.Matthay K.K. and Mentzer W.C. Erythrocyte enzymopathies in the newborn. Clin Haematol 10:31, 1981.
30.Smith G.D. and Vella F. Erythrocyte enzyme deficiency in unexplained kernicterus. Lancet 1:1133, 1960.
31.Bienzle U. Glucose 6-phosphate dehydrogenase deficiency. Part I : Tropical Africa. Clin Haematol 10:785, 1981.
32.Karayalcin C., Acs H. and Lanzkowsky P. G6PD deficiency and hyperbilinurinemia in black American full-term infants. NY State J Med 79:22, 1979.
33.Luzzatto L. Inherited haemolytic states : Glucose 6-phosphate dehydrogenase deficiency. Clin Hematol 4:83, 1975.
For more information on this disorder you can check the List of contraindicated drugs, the FAQ section and the Articles.
About us The Disorders The Products Kit Inserts Literature Technical Support Articles Events Links Contact us To Order
Hosted by
ohmywebsite.com
TO
SEARCH THIS SITE - CLICK
SEARCH
Page last edited on 05/07/2005