![]()
Prevention is better than Treatment
Hemoglobin Normalization (Hb+)
G-6-PD Diagnosis : Modification of the standard method eliminates the need for an additional Hemoglobin determination.
George J. Reclos Ph.D.1*.,Christine
J. Hatzidakis M.Sc.1 and Rudolf
A. Kruithof 2
1
R&D DIAGNOSTICS Ltd, 41 El.Venizelou Street, 15561 Holargos,
Greece
2 QUANTASE Limited,
3 Riverview Business Park, Friarton, Perth, PH2 8DF,
Scotland, UK
Greek Patent No. 1003227- International Patent Application No. NR PCT/GR99/00046/ 9/11/99
![]() Abstract
Abstract
Glucose- 6-Phosphate Dehydrogenase is one of the most common genetic diseases affecting an estimated 400.000.000 people worldwide. Neonatal screening for this disease has long been established in many countries and the method most commonly used is the semi-quantitative method described by Beutler or modifications to this method. Even when quantitative kits are used results are of little diagnostic value unless an assay to determine hemoglobin content is performed. This is time consuming and significantly increases the cost and the labor associated with the assay. On top of that, it doesn't solve the problem since any mistake during the actual G-6-PD assay, which could alter the Hb content, will not have been compensated for. Our work provides all the elements for a new approach measuring the Hb content in the same sample from which the enzyme activity is derived thus taking into account all mistakes or artifacts. The procedure is costless, fast, very simple, accurate and can be fully automated with almost any equipment.
![]() Περίληψη
Περίληψη
Η ανεπάρκεια του ενζύμου της αφυδρογονάσης της 6-φωσφορικής γλυκόζης (G-6-PD) είναι μία από τις πλέον κοινές γενετικές ασθένειες με περισσότερους από 400.000.000 πάσχοντες παγκοσμίως. Ο προληπτικός έλεγχος των νεογνών για αυτή την ασθένεια γίνεται σε πολλές χώρες εδώ και αρκετά χρόνια οι δε μέθοδοι που χρησιμοποιούνται είναι κυρίως παραλλαγές της ημιποσοτικής μεθόδου που περιγράφηκε από τον Beutler. Ακόμη και η χρήση ποσοτικών μεθόδων προσδιορισμού της δραστικότητας του ενζύμου αυτού έχουν πολύ μικρή διαγνωστική αξία αν δεν αυνοδεύονται από ποσοτικό προσδιορισμό της αιμοσφαιρίνης (συνήθως επιπρόσθετη γενική εξέταση αίματος).Αυτή η επιπλέον εξέταση αυξάνει σημαντικά το κόστος της εξέτασης, τον χρόνο και την εργασία που απαιτούνται για αυτή καθώς και τον χρόνο που χρειάζεται για να δοθεί ένα αξιόπιστο αποτέλεσμα. Επιπλέον, ακόμη και ο προσδιορισμός της αιμοσφαιρίνης των δειγμάτων δεν λύνει ολοκληρωτικά το πρόβλημα μιά και δεν μπορεί να διορθώσει οποιοδήποτε λάθος γίνει κατά την διάρκεια του προσδιορισμού της δραστικότητας του ενζύμου. Η εργασία αυτή παρέχει όλα τα στοιχεία για τον προσδιορισμό της περιεχόμενης αιμοσφαιρίνης μέσα στο ίδιο δείγμα στο οποίο γίνεται και ο προσδιορισμός του ενζύμου και λαμβάνει υπόψη της οποιοδήποτε λάθος μπορεί να γίνει κατά την εκτέλεση του προσδιορισμού. Η προτεινόμενη μέθοδος είναι ανέξοδη, ταχεία, απλή, ακριβής και μπορεί να αυτοματοποιηθεί πλήρως με κάθε εξοπλισμό. Η εργασία αυτή φιλοδοξεί να λύσει το πρόβλημα της μέτρησης της αιμοσφαιρίνης σε όλες τις μεθόδους οι οποίες μετρούν δραστικότητα ενζύμων ή γενικότερα αναλύτες οι οποίοι πρέπει να εκφραστούν συναρτήσει της εξετασθείσας ποσότητας ολικού αίματος.
Expressing
Results in Units / gram Hb.
The G-6-PD activity of the sample is now directly comparable to that of the
control (ΔODcontrol340nm/min).
Since the control's activity is already given in Units / g Hb (U/g Hb) the
sample's activity can be readily expressed in the same units. Special care
should be taken to use controls, which are rated, at the temperature used for
the assay. Thus, when using analyzers or microplate readers equipped with a
heating device, any control can be used. When performing the assay at room
temperature it is preferable to use Quantase controls which are rated at 24oC.
The equation that gives results expressed in U/g Hb is given in Table I.
(δODsample340nm/min)/(δODcontrol340nm/min)
-----------------------------------------------------------------------------
X
Control=Sample (U/g Hb)
ODsample405nm / ODcontrol405nm
The formula above was based on the finding that the Hb content measured at 405nm is always proportional to the actual Hb content when measured at the optimal wavelength (417 nm). This is shown in the graph below. It includes results from 50 samples both Dried Blood Spots (n=25) and whole blood samples (n=25).
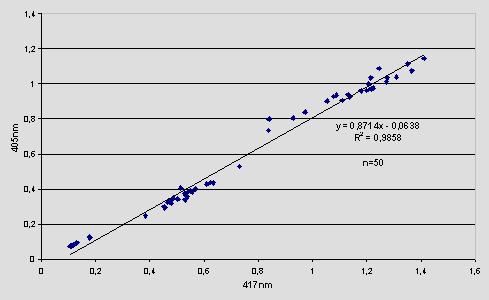
This approach should not be limited to G-6-PD screening only. Preliminary results have shown that it could be used in all assays involving a blood elution stage, since the results obtained correspond to the actual quantity of blood eluted. Moreover, cuttings are not always the same and many times a partially spotted paper disk may lead to erroneous results. The proposed extra reading of the microplate (or individual sample) at 405 nm can be performed at any convenient stage of a given method. Thus, when using methods that employ chemicals absorbing in this region (400-420nm) the extra reading should be taken immediately after the elution stage, before adding the chemicals. The same is true in cases where a color reagent is added or the final product of the assay absorbs in this wavelength range.
The following graph presents the data obtained by scanning the 580-323 nm range during the kinetic of a normal sample at 37oC. The sample was prepared as usually (according to the method for the MMR500 G-6-PD kit) but, instead of running the sample in kinetic mode, we chose the "scan" option. The complete range scanning takes 1 minute to complete (detail set to "coarse"). We used an electronic stopwatch to count the time intervals. The wavelengths of importance were the following : 323nm (lowest visible wavelength), 340nm (beta-NADPH optimum), 380nm, 405nm (proposed Hb evaluation), 414nm (optimum Hb wavelength), 450nm, 512nm (second Hb peak - not optimum) and 540nm (third Hb peak - not optimum). The x-axis (wavelengths) is not proportional in order to show more details in the range of interest (323 - 414 nm). The y axis shows the ODs.
SCANNING THE 323 - 540 nm WAVELENGTH RANGE

Comments : It is clear from the graph that the beta-NADPH produced during the kinetic does not affect the readings in the 400-430 nm wavelength range. This is also true for the chemicals (data not shown here) as measured before the addition of the sample. No chemical was shown to absorb in this range. In fact if blood is not present (G-6-PD from another source is used in an aqueous solution) this range shows no absorption at all. Blank : Dilution buffer. Reference : Hemoglobin solution diluted to the same final concentration as the sample with Elution and Dilution buffer. In conclusion, the only entity that absorbs in the 400-430 nm range is Hemoglobin.

Comments The extra OD reading at 405 nm was taken after termination of the kinetic reaction, as described in the Quantase MMR500 insert. No modifications of the standard method were introduced with the sole exception of the extra reading at 405 nm. It is clearly shown that the proposed extra measurement at 405 nm correlated very well with the actual Hemoglobin contained in the sample. This is a whole blood sample. When using dried blood spots, one can estimate to yield approximately half the Hb quantity contained in the spot. Our results have demonstrated that the same correlation is true for dried blood spot samples as well. Actually it is like viewing samples similar to the 50% samples of the graph above. For additional information please E-mail us to send you a full copy of our paper. Click next page for more information.
This work was published in Farmakeftiki.Click on the icon to get the full paper.
About us The Disorders The Products Kit Inserts Literature Technical Support Articles Events Links Contact us To Order
Hosted by
ohmywebsite.com
TO
SEARCH THIS SITE - CLICK
SEARCH
Page last edited on 05/07/2005